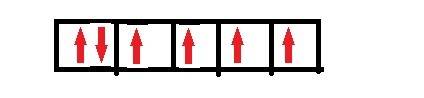
When transition metal atoms lose electrons to form ions, the electrons come first from the highest filled s atomic orbital. with this in mind , how many unpaired electrons are present in the fe2+ ion? hint: first write the outermost electron configuration of fe atom?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
When transition metal atoms lose electrons to form ions, the electrons come first from the highest f...
Questions

Mathematics, 21.12.2020 19:40

Mathematics, 21.12.2020 19:40

Advanced Placement (AP), 21.12.2020 19:40

Mathematics, 21.12.2020 19:40



Mathematics, 21.12.2020 19:40


Biology, 21.12.2020 19:40

Mathematics, 21.12.2020 19:40


Mathematics, 21.12.2020 19:40

Mathematics, 21.12.2020 19:40


Mathematics, 21.12.2020 19:40

Mathematics, 21.12.2020 19:40

Biology, 21.12.2020 19:40

Mathematics, 21.12.2020 19:40


History, 21.12.2020 19:40