
Be sure to answer all parts. industrially, hydrogen gas can be prepared by combining propane gas (c3h8) with steam at about 400°c. the products are carbon monoxide (co) and hydrogen gas (h2). (a) write a balanced equation for the reaction. include phase abbreviations. (b) how many kilograms of h2 can be obtained from 8.31 × 103 kg of propane

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3


Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
Be sure to answer all parts. industrially, hydrogen gas can be prepared by combining propane gas (c3...
Questions


Mathematics, 31.08.2020 15:01





Physics, 31.08.2020 15:01


English, 31.08.2020 15:01

Chemistry, 31.08.2020 15:01

History, 31.08.2020 15:01




English, 31.08.2020 15:01

Mathematics, 31.08.2020 15:01

Chemistry, 31.08.2020 15:01



Social Studies, 31.08.2020 15:01

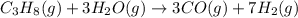
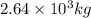
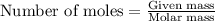
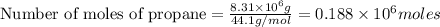
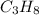 gives 7 moles of
gives 7 moles of 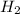
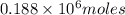 moles of
moles of 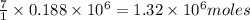 of
of 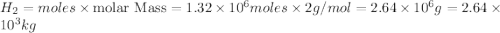
 kg of propane
kg of propane

