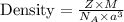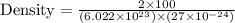Chemistry, 09.07.2019 17:00 jaallen3679
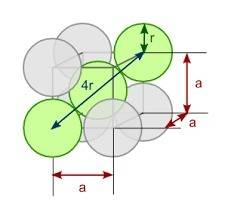
You discover a new alloy made up of cu and ni atoms. it has a bcc structure, where the ni atoms are located at the corners and the cu atoms are located in the center of each cell. the radius of the cu atoms is 0.13 nm and the radius of the ni atom is 0.15 nm. calculate the density of this structure. assume that cu has an atomic weight of 40 g/mol and ni has an atomic weight of 60 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3


Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

You know the right answer?
You discover a new alloy made up of cu and ni atoms. it has a bcc structure, where the ni atoms are...
Questions


Arts, 01.07.2019 06:50



History, 01.07.2019 06:50


Social Studies, 01.07.2019 06:50

English, 01.07.2019 06:50


Business, 01.07.2019 06:50



History, 01.07.2019 06:50

English, 01.07.2019 06:50


Social Studies, 01.07.2019 06:50

History, 01.07.2019 06:50


History, 01.07.2019 06:50

Mathematics, 01.07.2019 06:50

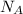 ) = 6.022 ×
) = 6.022 × 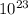
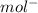
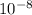 cm
cm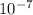 cm
cm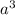 =
= 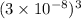 = 27 ×
= 27 × 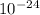
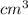
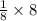 = 1atom of Ni and 1 atom of Cu
= 1atom of Ni and 1 atom of Cu