
Chemistry, 09.07.2019 17:30 1hannacarson
Consider the combustion of h2(g) 2h2(g)+o2(g)→2h2o(g). if hydrogen is burning at the rate of 0.49 mol/s, what is the rate of consumption of oxygen

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
Consider the combustion of h2(g) 2h2(g)+o2(g)→2h2o(g). if hydrogen is burning at the rate of 0.49 mo...
Questions











Mathematics, 31.03.2020 03:22


Mathematics, 31.03.2020 03:22

Mathematics, 31.03.2020 03:22






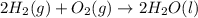
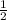 × 0.49 mol/s
× 0.49 mol/s

