
Chemistry, 09.07.2019 17:30 megansanders215
Consider the reaction below: a (aq) ↔ b (aq) kc = 2.36 if the reaction is started by placing 0.134 mol of a into 250.0 ml of solution, what will the concentration of a be at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
Consider the reaction below: a (aq) ↔ b (aq) kc = 2.36 if the reaction is started by placing 0.134...
Questions


History, 27.05.2021 01:00

Mathematics, 27.05.2021 01:10


English, 27.05.2021 01:10

History, 27.05.2021 01:10

Mathematics, 27.05.2021 01:10

Mathematics, 27.05.2021 01:10

Mathematics, 27.05.2021 01:10


Spanish, 27.05.2021 01:10



Mathematics, 27.05.2021 01:10

English, 27.05.2021 01:10

Mathematics, 27.05.2021 01:10

Geography, 27.05.2021 01:10

Mathematics, 27.05.2021 01:10

Mathematics, 27.05.2021 01:10

Chemistry, 27.05.2021 01:10

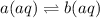
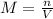
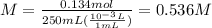
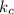 for the equilibrium reaction is:
for the equilibrium reaction is:![k_{c}=\frac{[b]}{[a]}](/tpl/images/0070/2226/ea1a2.png)
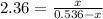
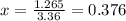
![[a]=0.536-x=0.536-0.376=0.16 M](/tpl/images/0070/2226/22b1e.png)



