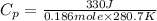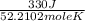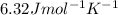Chemistry, 09.07.2019 21:00 maxi12312345
It takes 330 j of energy to raise the temperature of 14.6 g of benzene from 21.0 °c to 28.7 °c at constant pressure. what is the constant-pressure molar heat capacity of benzene?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2


Chemistry, 23.06.2019 11:50
How many moles of an ideal gas would occupy a 25.0 liter container when the temperature is 295 k and the pressure is 0.850 atm?
Answers: 2

You know the right answer?
It takes 330 j of energy to raise the temperature of 14.6 g of benzene from 21.0 °c to 28.7 °c at co...
Questions












Mathematics, 08.10.2019 16:20






Business, 08.10.2019 16:20

English, 08.10.2019 16:20

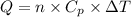 (1)
(1)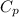 = molar heat capacity at constant pressure
= molar heat capacity at constant pressure = change in temperature
= change in temperature 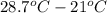
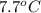
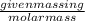
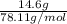
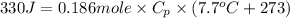 (conversion of degree Celsius into kelvin)
(conversion of degree Celsius into kelvin)