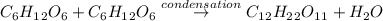Chemistry, 09.07.2019 21:00 queenpanda365
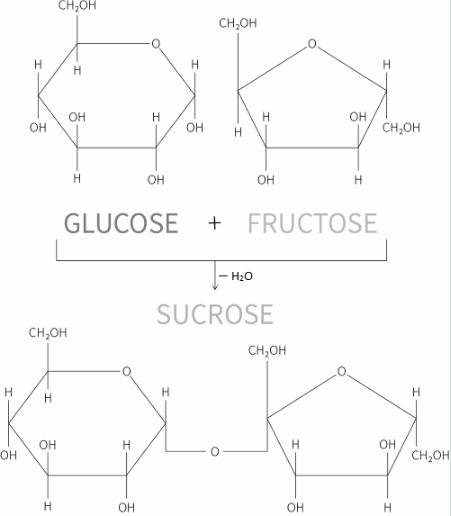
If sucrose (c12h22o11) is composed of glucose (c6h12o6) and fructose (c6h12o6) shouldn't the molecular formula be (c12h24o12). what happened to the missing hydrogen and oxygen atoms?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
If sucrose (c12h22o11) is composed of glucose (c6h12o6) and fructose (c6h12o6) shouldn't the molecul...
Questions

Mathematics, 06.05.2020 08:12


Chemistry, 06.05.2020 08:13




Medicine, 06.05.2020 08:13



Mathematics, 06.05.2020 08:13

Mathematics, 06.05.2020 08:13




Chemistry, 06.05.2020 08:13

Mathematics, 06.05.2020 08:13

English, 06.05.2020 08:13


Spanish, 06.05.2020 08:13

Mathematics, 06.05.2020 08:13

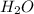 ,
, 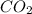 etc. as a byproduct.
etc. as a byproduct. 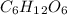 and Fructose(
and Fructose(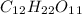 ) and release water(
) and release water(