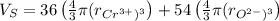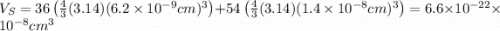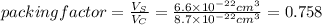Chemistry, 09.07.2019 22:00 nancye2008
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 nm. if the density of this material is 5.22 g/cm3, calculate its atomic packing factor. the atomic weights of cr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2

Chemistry, 23.06.2019 07:30
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2
You know the right answer?
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 n...
Questions

Physics, 08.03.2021 20:50

Chemistry, 08.03.2021 20:50




History, 08.03.2021 20:50

English, 08.03.2021 20:50

Mathematics, 08.03.2021 20:50




Mathematics, 08.03.2021 20:50


Mathematics, 08.03.2021 20:50

Biology, 08.03.2021 20:50


Mathematics, 08.03.2021 20:50


English, 08.03.2021 20:50

Mathematics, 08.03.2021 20:50

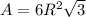
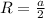
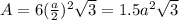
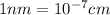
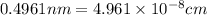
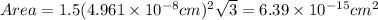
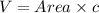
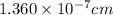
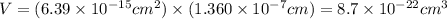
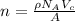
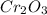 is 151.99 g/mol.
is 151.99 g/mol.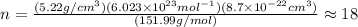
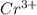 and
and 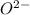 is 62 pm and 140 pm respectively.
is 62 pm and 140 pm respectively. 
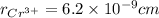
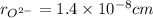
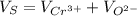
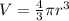 ,
,