
Chemistry, 10.07.2019 00:30 chrisraptorofficial
Which statement about reversible reactions is correct? at equilibrium, the forward and reverse reactions stop at the appropriate concentrations. at equilibrium, the forward and reverse reactions continue indefinitely. at equilibrium the rate of reaction of products divided by the rate of reaction of reactants equal the equilibrium constant, k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3


Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
Which statement about reversible reactions is correct? at equilibrium, the forward and reverse reac...
Questions

Mathematics, 02.01.2021 18:10

Mathematics, 02.01.2021 18:10





Chemistry, 02.01.2021 18:20




Mathematics, 02.01.2021 18:20



Social Studies, 02.01.2021 18:20

Biology, 02.01.2021 18:20

Social Studies, 02.01.2021 18:20




Business, 02.01.2021 18:20

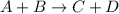
![k = \frac{[C][D]}{[A][B]}](/tpl/images/0071/3436/05b05.png)
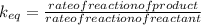
![\frac{\frac{d[C][D]}{dt}}{\frac{d[A][B]}{dt}}](/tpl/images/0071/3436/6ae5f.png)


