Chemistry, 10.07.2019 01:30 isaiahmichel93081
3: you place 2.56 g of caco3(fw = 100.09 g/mol) in a beaker containing 250. ml of 0.125 m hcl. what the reactions has ceased, what mass of cacl2(fw = 110.98 g/mol) can be produced? the unbalancedequation i

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

You know the right answer?
3: you place 2.56 g of caco3(fw = 100.09 g/mol) in a beaker containing 250. ml of 0.125 m hcl. what...
Questions



English, 28.12.2019 04:31

Chemistry, 28.12.2019 04:31

Geography, 28.12.2019 04:31

Biology, 28.12.2019 04:31


Mathematics, 28.12.2019 04:31



History, 28.12.2019 04:31


English, 28.12.2019 04:31




History, 28.12.2019 04:31

Biology, 28.12.2019 04:31

Mathematics, 28.12.2019 04:31

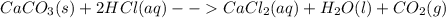
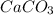 =
=
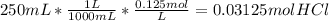
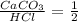
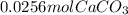 per 0.03125 mol HCl
per 0.03125 mol HCl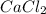 =0.03125mol HCl *
=0.03125mol HCl *