
Chemistry, 10.07.2019 03:30 jadeandryna0609
The vapor pressure of benzene, c6h6, is 100.0 torr at 26.1 °c. assuming raoult's law is obeyed, how many moles of a nonvolatile solute must be added to 100.0 ml of benzene to decrease its vapor pressure by 10.0% at 26.1 °c? the density of benzene is 0.8765 g> cm3.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
The vapor pressure of benzene, c6h6, is 100.0 torr at 26.1 °c. assuming raoult's law is obeyed, how...
Questions

Mathematics, 20.11.2020 05:40




Mathematics, 20.11.2020 05:40




Mathematics, 20.11.2020 05:40

Mathematics, 20.11.2020 05:40

Biology, 20.11.2020 05:40


English, 20.11.2020 05:40


Mathematics, 20.11.2020 05:40

History, 20.11.2020 05:40


Mathematics, 20.11.2020 05:40


Mathematics, 20.11.2020 05:40

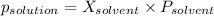 -(1)
-(1)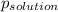 is observed vapor pressure of the solution,
is observed vapor pressure of the solution, 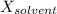 is mole fraction of solvent, and
is mole fraction of solvent, and 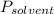 is vapor pressure of the pure solvent.
is vapor pressure of the pure solvent.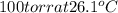 (given)
(given)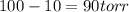
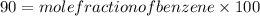
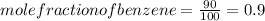
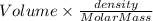
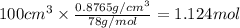
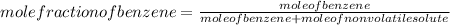
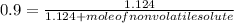
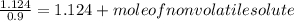
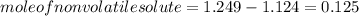
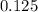 .
.

