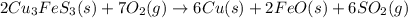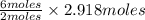Chemistry, 10.07.2019 03:30 astepania0003
Using the balanced chemical reaction below, how many grams of copper (cu) metal can be obtained from 1.00 kg of copper ore (cu3fes3)? 2cu3fes3 (s) + 7o2 (g) ; → 6cu (s) + 2feo (s) + 6so2 (g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Using the balanced chemical reaction below, how many grams of copper (cu) metal can be obtained from...
Questions




Mathematics, 09.04.2021 19:50


Mathematics, 09.04.2021 19:50


Social Studies, 09.04.2021 19:50

Mathematics, 09.04.2021 19:50

History, 09.04.2021 19:50

Mathematics, 09.04.2021 19:50



Spanish, 09.04.2021 19:50


Mathematics, 09.04.2021 19:50

Mathematics, 09.04.2021 19:50


History, 09.04.2021 19:50

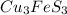 = 342.678 g/mole
= 342.678 g/mole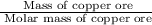 =
= 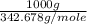 = 2.918 moles
= 2.918 moles