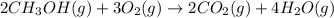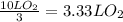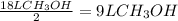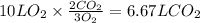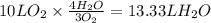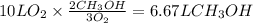Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3
You know the right answer?
Consider the combustion of methanol at some high temperature in a constant-pressure reaction chamber...
Questions


Mathematics, 11.06.2020 00:57


Mathematics, 11.06.2020 00:57


Mathematics, 11.06.2020 00:57

Mathematics, 11.06.2020 00:57

Mathematics, 11.06.2020 00:57

Mathematics, 11.06.2020 00:57

Computers and Technology, 11.06.2020 00:57





Biology, 11.06.2020 00:57

Mathematics, 11.06.2020 00:57


English, 11.06.2020 00:57