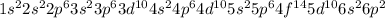Chemistry, 10.07.2019 04:00 mcgowans348
Determine the quantum numbers n and l of the electron removed when bi is ionized to bi+

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 21:00
Imagine that you are buying books one by one and then piling them up after reading them. which geologic concept could you use to identify the topmost book as the newest?
Answers: 3

Chemistry, 23.06.2019 21:50
Draw the lewis structure of ph3. to add lone pairs, click the button before clicking on the molecule. draw the molecule by placing atoms on the grid and connecting them with bonds. include lone pairs of electrons and hydrogen atoms. view available hint(s)
Answers: 1
You know the right answer?
Determine the quantum numbers n and l of the electron removed when bi is ionized to bi+...
Questions


Mathematics, 10.08.2021 01:40




Mathematics, 10.08.2021 01:40

Mathematics, 10.08.2021 01:40

Computers and Technology, 10.08.2021 01:40




Mathematics, 10.08.2021 01:40

Computers and Technology, 10.08.2021 01:40

Social Studies, 10.08.2021 01:40

Mathematics, 10.08.2021 01:40

Mathematics, 10.08.2021 01:40




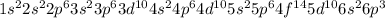
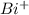 , the electronic configuration becomes:
, the electronic configuration becomes: