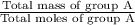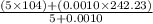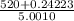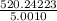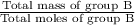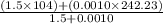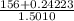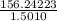Chemistry, 10.07.2019 05:30 elijahjacksonrp6z2o7
Group a used 5.00 moles of styrene and 0.0010 moles of benzoyl peroxide while group b used the same amount of benzoyl peroxide as group a but used only 1.50 moles styrene. what would the average molecular weight of each group's polymer sample be

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
Group a used 5.00 moles of styrene and 0.0010 moles of benzoyl peroxide while group b used the same...
Questions


Mathematics, 20.11.2020 22:10


Chemistry, 20.11.2020 22:10

Biology, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10

Social Studies, 20.11.2020 22:10



Mathematics, 20.11.2020 22:10


Mathematics, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10

History, 20.11.2020 22:10



Mathematics, 20.11.2020 22:10