

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Gthe normal range of the ph of blood is between 7.35 and 7.45; variations beyond this range have si...
Questions

Mathematics, 30.12.2020 05:50

Mathematics, 30.12.2020 05:50

Mathematics, 30.12.2020 05:50


Mathematics, 30.12.2020 05:50



Mathematics, 30.12.2020 05:50

Biology, 30.12.2020 05:50





Mathematics, 30.12.2020 05:50

Physics, 30.12.2020 05:50




English, 30.12.2020 05:50

Business, 30.12.2020 05:50

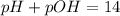
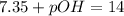
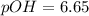
![pOH = - log [OH-]](/tpl/images/0072/1815/088b1.png)
![[OH-] = 10^{-6.65}](/tpl/images/0072/1815/b0e09.png)
![[OH-] = 2.24 \times 10^{-7}](/tpl/images/0072/1815/80591.png)
![[H+] [OH-] = 1 \times 10^{-14}](/tpl/images/0072/1815/cc1b6.png)
![[H+] = \frac{1 \times 10^{-14}}{2.24 \times 10^-7} = 4.46 \times 10^{-8} M](/tpl/images/0072/1815/f5927.png)
![[H+] = \frac{1 \times 10^{-14}}{2.82 \times 10^-7} = 3.54 \times 10^{-8} M](/tpl/images/0072/1815/3f0d8.png)


