
Chemistry, 10.07.2019 06:00 xbeatdroperzx
Phenol, c6h5oh, is a stronger acid than methanol, ch3oh, even though both contain an o]h bond. draw the structures of the anions resulting from loss of h1 from phenol and methanol, and use resonance structures to explain the difference in acidity.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Phenol, c6h5oh, is a stronger acid than methanol, ch3oh, even though both contain an o]h bond. draw...
Questions

Computers and Technology, 05.03.2021 14:00







Mathematics, 05.03.2021 14:00

Business, 05.03.2021 14:00

Business, 05.03.2021 14:00

Mathematics, 05.03.2021 14:00

Mathematics, 05.03.2021 14:00

Chemistry, 05.03.2021 14:00

English, 05.03.2021 14:00

Chemistry, 05.03.2021 14:00

Mathematics, 05.03.2021 14:00

Mathematics, 05.03.2021 14:00

Mathematics, 05.03.2021 14:00

Social Studies, 05.03.2021 14:00

Arts, 05.03.2021 14:00

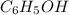 and methanol,
and methanol, 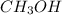 both are alcohols that contain an
both are alcohols that contain an 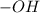 group attached to carbon atom.
group attached to carbon atom.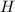 from phenol, it forms phenoxide anion and due to presence of double bond in the benzene ring the negative charge on the oxygen atom (which represents electrons) will resonate with double bonds of benzene ring as shown in the image. The resonance-stabilized phenoxide ion is more stable. Whereas when methanol lose 1
from phenol, it forms phenoxide anion and due to presence of double bond in the benzene ring the negative charge on the oxygen atom (which represents electrons) will resonate with double bonds of benzene ring as shown in the image. The resonance-stabilized phenoxide ion is more stable. Whereas when methanol lose 1 ![Phenol, c6h5oh, is a stronger acid than methanol, ch3oh, even though both contain an o]h bond. draw](/tpl/images/0072/2100/80d06.jpg)


