Chemistry, 10.07.2019 06:00 Meap12345678910
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 3.75 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 3.75 ∘c. what is the molal concentration...
Questions


History, 10.03.2020 09:27

Computers and Technology, 10.03.2020 09:28





Physics, 10.03.2020 09:28

Mathematics, 10.03.2020 09:29


Mathematics, 10.03.2020 09:29

Mathematics, 10.03.2020 09:29








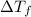 can be calculated as follows:
can be calculated as follows: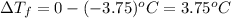
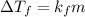
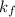 is freezing point depression constant.
is freezing point depression constant.