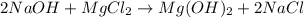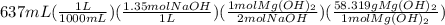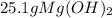Sodium hydroxide and magnesium chloride react as shown by this equation: 2naoh + mgcl2 → mg(oh)2 + 2nacl. suppose the reaction begins with 637 milliliters of 1.35 m sodium hydroxide solution and excess magnesium hydroxide. what is the theoretical yield of magnesium hydroxide if the resulting solution has a volume of 2.82 liters? use the periodic table and the polyatomic ion resource. the mass of magnesium hydroxide formed is grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Sodium hydroxide and magnesium chloride react as shown by this equation: 2naoh + mgcl2 → mg(oh)2 +...
Questions

Biology, 08.07.2019 15:30

English, 08.07.2019 15:30

English, 08.07.2019 15:30




Mathematics, 08.07.2019 15:30

Mathematics, 08.07.2019 15:30


History, 08.07.2019 15:30


Biology, 08.07.2019 15:30



Business, 08.07.2019 15:30

Biology, 08.07.2019 15:30

Physics, 08.07.2019 15:30

History, 08.07.2019 15:30

Health, 08.07.2019 15:30

Physics, 08.07.2019 15:30