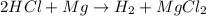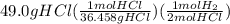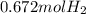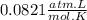When magnesium reacts with hydrochloric acid, hydrogen gas is formed: 2hcl + mg → h2 + mgcl2. what is the volume of hydrogen produced at 25°c and 101.3 kilopascals when 49.0 grams of hcl reacts with excess magnesium? use the periodic table and ideal gas resource. a. 1.38 l b. 2.76 l c. 16.4 l d. 32.9 l e. 33.1 l

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
When magnesium reacts with hydrochloric acid, hydrogen gas is formed: 2hcl + mg → h2 + mgcl2. what...
Questions


Social Studies, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01




Mathematics, 10.09.2020 02:01

Computers and Technology, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01


English, 10.09.2020 02:01


Social Studies, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

History, 10.09.2020 02:01


Physics, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01