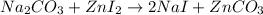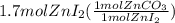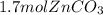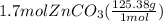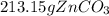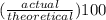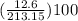Chemistry, 10.07.2019 07:30 takaralocklear
Areaction between 1.7 moles of zinc iodide and excess sodium carbonate yields 12.6 grams of zinc carbonate. this is the equation for the reaction: na2co3 + zni2 → 2nai + znco3. what is the percent yield of zinc carbonate? the percent yield of zinc carbonate is %.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3
You know the right answer?
Areaction between 1.7 moles of zinc iodide and excess sodium carbonate yields 12.6 grams of zinc car...
Questions


Mathematics, 18.09.2019 21:30


History, 18.09.2019 21:30





Computers and Technology, 18.09.2019 21:30