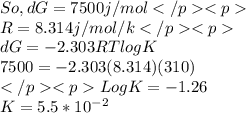Chemistry, 10.07.2019 08:30 sweetluvs7865
Calculate the equilibrium constant, and the equilibrium fraction of gap from the above, at 37 ∘c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
Calculate the equilibrium constant, and the equilibrium fraction of gap from the above, at 37 ∘c....
Questions


English, 10.06.2021 01:20

Mathematics, 10.06.2021 01:20

History, 10.06.2021 01:20


English, 10.06.2021 01:20

Biology, 10.06.2021 01:20

Physics, 10.06.2021 01:20

Biology, 10.06.2021 01:20


Computers and Technology, 10.06.2021 01:20


Mathematics, 10.06.2021 01:20

Social Studies, 10.06.2021 01:20

Mathematics, 10.06.2021 01:20

Social Studies, 10.06.2021 01:20



Social Studies, 10.06.2021 01:20

Mathematics, 10.06.2021 01:20