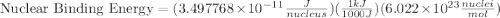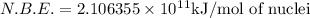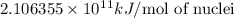Chemistry, 10.07.2019 12:30 janicemaxwell123
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and neutrons if an aluminum-27 atom has a mass of 26.9815386 amu? (the mass of an electron is 5.485799×10−4 amu, the mass of a proton is 1.0072765 amu, and the mass of a neutron is 1.0086649 amu.) express your answer using six significant figures. g?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and ne...
Questions


Advanced Placement (AP), 23.10.2019 07:00

Mathematics, 23.10.2019 07:00



History, 23.10.2019 07:00

English, 23.10.2019 07:00

English, 23.10.2019 07:00



Mathematics, 23.10.2019 07:00

History, 23.10.2019 07:00


Mathematics, 23.10.2019 07:00


History, 23.10.2019 07:00


Advanced Placement (AP), 23.10.2019 07:00


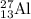 nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from
nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from 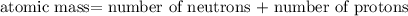 .
. 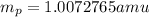 and
and 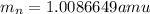 , we can calculate the mass of the isotope.
, we can calculate the mass of the isotope.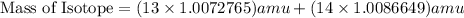
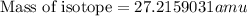
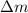 = mass of isotope - atomic mass.
= mass of isotope - atomic mass.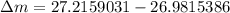
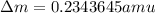
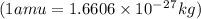
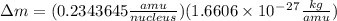
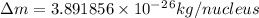
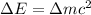
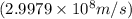
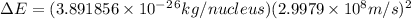
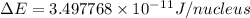
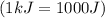 and converting individual particles into moles, we need to multiply it by avagadro's number that is
and converting individual particles into moles, we need to multiply it by avagadro's number that is 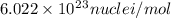 .
.