
Chemistry, 10.07.2019 15:00 hannahbanana2000
Which element does lithium (li) share more properties with, beryllium (be) or sodium (na)? lithium has more common properties with beryllium than sodium, because they have the same number of electron shells. lithium has more common properties with sodium than beryllium, because they have the same number of valence electrons. lithium has more common properties with beryllium than sodium, because they have the same number of valence electrons. lithium has more common properties with sodium than beryllium, because they have the same number of electron shells.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
Which element does lithium (li) share more properties with, beryllium (be) or sodium (na)? lithium h...
Questions


English, 09.07.2019 20:30


Spanish, 09.07.2019 20:30

Mathematics, 09.07.2019 20:30


Biology, 09.07.2019 20:30

Physics, 09.07.2019 20:30

History, 09.07.2019 20:30

Chemistry, 09.07.2019 20:30








Chemistry, 09.07.2019 20:30

Mathematics, 09.07.2019 20:30

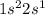 . This element belongs to Group 1 of the periodic table.
. This element belongs to Group 1 of the periodic table.


