Chemistry, 10.07.2019 17:00 sanders183
Gallium has two naturally occurring isotopes: 69ga with a mass of 68.9256 amu and a natural abundance of 60.11% and 71ga. use the atomic mass of gallium from the periodic table to find the mass of gallium-71. express the mass in atomic mass units to two de

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1


Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
Gallium has two naturally occurring isotopes: 69ga with a mass of 68.9256 amu and a natural abundan...
Questions



Computers and Technology, 17.11.2020 23:40

Computers and Technology, 17.11.2020 23:40

Arts, 17.11.2020 23:40


Biology, 17.11.2020 23:40

Mathematics, 17.11.2020 23:40



English, 17.11.2020 23:40




Mathematics, 17.11.2020 23:40


Mathematics, 17.11.2020 23:40



Mathematics, 17.11.2020 23:40

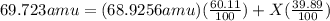
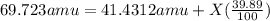
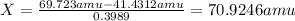
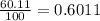
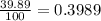
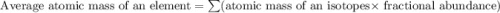
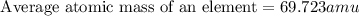
![69.723 =\sum[(68.9256\times 0.6011)+(x\times 0.3989)]](/tpl/images/0073/9705/d3b39.png)