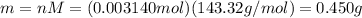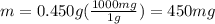Chemistry, 10.07.2019 17:30 icantspeakengles
Silver chloride, often used in silver plating, contains 75.27% ag. calculate the mass of silver chloride required to plate 255 mg of pure silver.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
Silver chloride, often used in silver plating, contains 75.27% ag. calculate the mass of silver chlo...
Questions



Computers and Technology, 19.09.2019 14:10



English, 19.09.2019 14:10

Biology, 19.09.2019 14:10

Biology, 19.09.2019 14:10




Biology, 19.09.2019 14:10

Physics, 19.09.2019 14:10




Mathematics, 19.09.2019 14:10



Biology, 19.09.2019 14:10

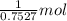 of AgCl.
of AgCl.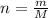
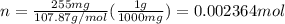
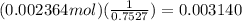 mol of AgCl.
mol of AgCl.