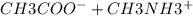Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
Acetic acid, ch3cooh, is a weak organic acid, pka 4.47. determine the position of the equilibrium fo...
Questions

Mathematics, 15.12.2020 21:50

Chemistry, 15.12.2020 21:50

Mathematics, 15.12.2020 21:50


Computers and Technology, 15.12.2020 21:50


Mathematics, 15.12.2020 21:50

Mathematics, 15.12.2020 21:50



Mathematics, 15.12.2020 21:50

Advanced Placement (AP), 15.12.2020 21:50

Mathematics, 15.12.2020 21:50


Mathematics, 15.12.2020 21:50

History, 15.12.2020 21:50




Arts, 15.12.2020 21:50

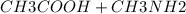 ↔
↔