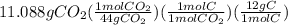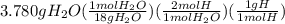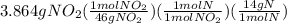Chemistry, 10.07.2019 21:00 jadynnnnn1795
Combustion of 8.652 g of a compound containing c, h, o, and n yields 11.088 g of coz, 3.780 g of h2o, and 3.864 g of no2. how many grams of c, h, and n, are contained in the sample?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Combustion of 8.652 g of a compound containing c, h, o, and n yields 11.088 g of coz, 3.780 g of h2o...
Questions





Mathematics, 04.03.2021 23:20



Computers and Technology, 04.03.2021 23:20


Mathematics, 04.03.2021 23:20

Mathematics, 04.03.2021 23:20

Mathematics, 04.03.2021 23:20

History, 04.03.2021 23:20


History, 04.03.2021 23:20

Advanced Placement (AP), 04.03.2021 23:20

Mathematics, 04.03.2021 23:20

Mathematics, 04.03.2021 23:20