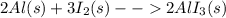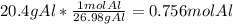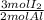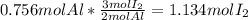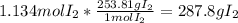Chemistry, 10.07.2019 21:00 greeneashlynt
Calculate the mass in grams of iodine (i2) that will react completely with 20.4 g of aluminum (al) to form aluminum iodide (ali3)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Calculate the mass in grams of iodine (i2) that will react completely with 20.4 g of aluminum (al) t...
Questions

Arts, 31.03.2021 01:40


Arts, 31.03.2021 01:40

Mathematics, 31.03.2021 01:40


Mathematics, 31.03.2021 01:40


Mathematics, 31.03.2021 01:40

Mathematics, 31.03.2021 01:40


English, 31.03.2021 01:40





Mathematics, 31.03.2021 01:40


History, 31.03.2021 01:40

Chemistry, 31.03.2021 01:40

Mathematics, 31.03.2021 01:40