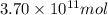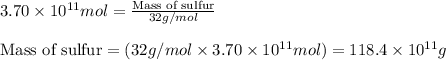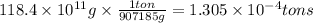Chemistry, 10.07.2019 21:00 hapjajsjjz3738
The annual production of sulfur dioxide from burning coal and fossil fuels, auto exhaust, and other sources is about 26 million tons. the equation for the reaction is s(s) + o2(g) → so2(g) how much sulfur (in tons), present in the original materials, would result in that quantity of so2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
The annual production of sulfur dioxide from burning coal and fossil fuels, auto exhaust, and other...
Questions



Biology, 10.12.2019 08:31




Mathematics, 10.12.2019 08:31


History, 10.12.2019 08:31


History, 10.12.2019 08:31

Mathematics, 10.12.2019 08:31

Mathematics, 10.12.2019 08:31


Mathematics, 10.12.2019 08:31


Mathematics, 10.12.2019 08:31

Mathematics, 10.12.2019 08:31


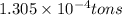
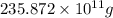 (Conversion factor:
(Conversion factor: 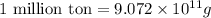 )
)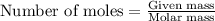 .....(1)
.....(1)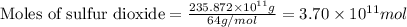
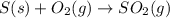
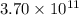 moles of sulfur dioxide will be produced by =
moles of sulfur dioxide will be produced by = 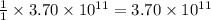 moles of sulfur.
moles of sulfur.