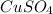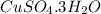Chemistry, 10.07.2019 21:00 saltedcaramel60
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of attached water molecules. after heating the hydrate, you have 3.3608 g of the anhydrous compound (copper(ii) sulfate with no waters) left. using these data, calculate the number of water molecules that is present in the formula of this hydrate (obviously before heating).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1


Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of a...
Questions

Mathematics, 20.07.2021 22:20

Mathematics, 20.07.2021 22:20

English, 20.07.2021 22:20




Mathematics, 20.07.2021 22:20



Chemistry, 20.07.2021 22:20

Mathematics, 20.07.2021 22:20


Biology, 20.07.2021 22:20

History, 20.07.2021 22:20





Chemistry, 20.07.2021 22:20

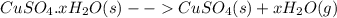
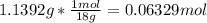
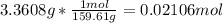
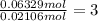
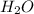 per one mol
per one mol