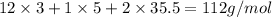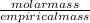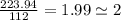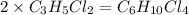Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
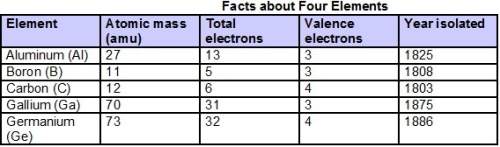
An unknown compound with a molar mass of 223.94 g/mol consists of 32.18% c, 4.50% h, and 63.32% cl....
Questions

Mathematics, 30.08.2020 01:01



Advanced Placement (AP), 30.08.2020 01:01

Biology, 30.08.2020 01:01




English, 30.08.2020 01:01

Social Studies, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01


Mathematics, 30.08.2020 01:01

Spanish, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01

English, 30.08.2020 01:01

History, 30.08.2020 01:01

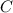 :
: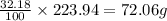
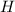 :
: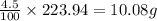
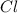 :
: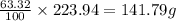
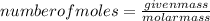
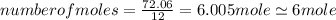
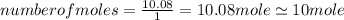
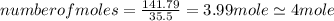
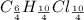
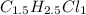
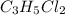 .
.