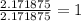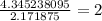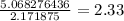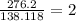Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
Acompound sample contains 60.87% c, 4.38% h, and 34.75% o by mass. it has a molar mass of 276.2 g/mo...
Questions


Mathematics, 25.07.2019 07:20



Mathematics, 25.07.2019 07:20


Mathematics, 25.07.2019 07:20








English, 25.07.2019 07:20

Social Studies, 25.07.2019 07:20


Mathematics, 25.07.2019 07:20


Mathematics, 25.07.2019 07:20