
This reaction shows the complete combustion of octane, c8h18, a component of gasoline. 2 c8h18(l) + 25 o2(g) 16 co2(g) + 18 h2o(l) (a) how many moles of o2 are needed to burn 1.60 mol of c8h18? mol (b) how many moles of co2 are produced when 0.19 mol of c8h18 are burned? mol (c) how many grams of o2 are needed to burn 1.15 g of c8h18? g

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 06:30
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2
You know the right answer?
This reaction shows the complete combustion of octane, c8h18, a component of gasoline. 2 c8h18(l) +...
Questions


Mathematics, 17.04.2021 03:20



Chemistry, 17.04.2021 03:20

Mathematics, 17.04.2021 03:20

English, 17.04.2021 03:20

Mathematics, 17.04.2021 03:20

History, 17.04.2021 03:20

Advanced Placement (AP), 17.04.2021 03:20

Mathematics, 17.04.2021 03:20




Chemistry, 17.04.2021 03:20





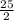 O₂→ 8 CO₂ + 9 H₂O.
O₂→ 8 CO₂ + 9 H₂O. 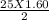 mole= 20 moles.
mole= 20 moles.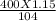 g= 4.42 g.
g= 4.42 g.

