
Chemistry, 11.07.2019 11:30 LindaCat78
Zinc metal reacts with hydrochloric acid according to the balanced equation: zn(s) + 2 hcl(aq) ¡ zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.5 °c to 23.7 °c. find ∆hrxn for this reaction as written. (use 1.0 g/ml for the

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1


Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Zinc metal reacts with hydrochloric acid according to the balanced equation: zn(s) + 2 hcl(aq) ¡ zn...
Questions

English, 30.09.2020 01:01

Chemistry, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01

English, 30.09.2020 01:01



Mathematics, 30.09.2020 01:01


Arts, 30.09.2020 01:01


Mathematics, 30.09.2020 01:01




Mathematics, 30.09.2020 01:01




English, 30.09.2020 01:01

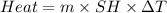
 is change in temperature.
is change in temperature.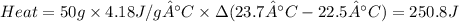
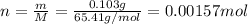
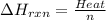
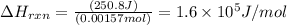
 for the given reaction is
for the given reaction is 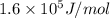
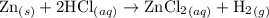
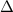 T,
T,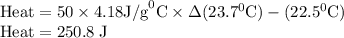
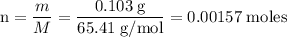
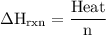
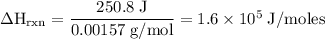
 =
= 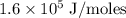 for the given equation.
for the given equation. 

