
Chemistry, 11.07.2019 15:00 wittlemarie
At a certain temperature, the solubility of n2 gas in water at 3.08 atm is 72.5 mg of n2 gas/100 g water . calculate the solubility of n2 gas in water, at the same temperature, if the partial pressure of n2 gas over the solution is increased from 3.08 atm to 8.00 atm . express your answer numerically to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
At a certain temperature, the solubility of n2 gas in water at 3.08 atm is 72.5 mg of n2 gas/100 g w...
Questions




Mathematics, 13.07.2021 21:40

Mathematics, 13.07.2021 21:40




Mathematics, 13.07.2021 21:40




Spanish, 13.07.2021 21:40

History, 13.07.2021 21:40

Mathematics, 13.07.2021 21:40


Mathematics, 13.07.2021 21:40

Mathematics, 13.07.2021 21:40

Chemistry, 13.07.2021 21:40

Arts, 13.07.2021 21:40


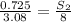
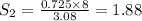
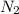 gas in 1 g of water or, 188 mg of tex]N_{2}[/tex] gas in 100 g of water.
gas in 1 g of water or, 188 mg of tex]N_{2}[/tex] gas in 100 g of water.

