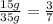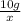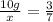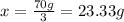Two samples of a compound containing elements a and b are decomposed. the first sample produces 15 g of a and 35 g of b. you may want to reference ( page 49) section 2.3 while completing this problem. part a the second sample produces 10 g of a and what mass of b? the second sample produces 10 of and what mass of ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Two samples of a compound containing elements a and b are decomposed. the first sample produces 15 g...
Questions


Mathematics, 04.11.2019 01:31

History, 04.11.2019 01:31

Mathematics, 04.11.2019 01:31

Biology, 04.11.2019 01:31








English, 04.11.2019 01:31

Social Studies, 04.11.2019 01:31

Mathematics, 04.11.2019 02:31

Mathematics, 04.11.2019 02:31

History, 04.11.2019 02:31

Mathematics, 04.11.2019 02:31


Mathematics, 04.11.2019 02:31