
Bromine reacts with nitric oxide to form nitrosyl bromide as shown in this reaction: br2(g) + 2 no(g) → 2 nobr(g) a possible mechanism for this overall reaction is shown below. no(g) + br2(g) br2(g) (fast step; keq = k1/k−1) k2 nobr(g) + no(g) → 2 nobr(g) (slow step) what is the rate law for formation of nobr in terms of reactants based on this mechanism

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
Bromine reacts with nitric oxide to form nitrosyl bromide as shown in this reaction: br2(g) + 2 no(...
Questions

Mathematics, 05.05.2020 01:00

Chemistry, 05.05.2020 01:00

Social Studies, 05.05.2020 01:00


Mathematics, 05.05.2020 01:00

English, 05.05.2020 01:00




Mathematics, 05.05.2020 01:00

English, 05.05.2020 01:00

Mathematics, 05.05.2020 01:00



Advanced Placement (AP), 05.05.2020 01:00


Mathematics, 05.05.2020 01:00



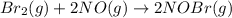
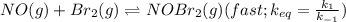
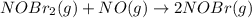 (slow step
(slow step 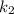 )
)![r_{1}=k_{1}[NO][Br_{2}]-k_{-1}[NOBr_{2}]](/tpl/images/0077/5023/9bc26.png)
![r_{2}=k_{2}[NOBr_{2}] [NO]](/tpl/images/0077/5023/6e659.png)
![[NOBr_{2}]](/tpl/images/0077/5023/48931.png) takes place in this reaction.
takes place in this reaction.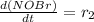
![k_{2}[NOBr_{2}][NO]](/tpl/images/0077/5023/433e8.png) (1)
(1)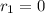
![k_{1}[NO][Br_{2}]= k_{-1}[NOBr_{2}]](/tpl/images/0077/5023/8345a.png)
![[NOBr_{2}] = \frac{k_{1}}{k_{-1}}[NO][Br_{2}]](/tpl/images/0077/5023/ee42f.png)
![\frac{d(NOBr)}{dt}=k_{2}[NOBr_{2}][NO]](/tpl/images/0077/5023/ff99b.png)
![k_{2} \frac{k_{1}}{k_{-1}}[NO][Br_{2}][NO]](/tpl/images/0077/5023/f5111.png)
![\frac{k_{1}k_{2}}{k_{-1}}[NO]^{2}[Br_{2}]](/tpl/images/0077/5023/427a8.png)
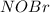 in terms of reactants is given by
in terms of reactants is given by 


