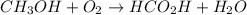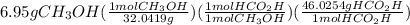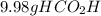Chemistry, 11.07.2019 16:00 hazeleyes2006
For the reaction ? ch3oh+? o2 →? hco2h+? h2o , what is the maximum amount of hco2h (46.0254 g/mol) which could be formed from 6.95 g of ch3oh (32.0419 g/mol) and 8.11 g of o2 (31.9988 g/mol)? answer in units of g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
For the reaction ? ch3oh+? o2 →? hco2h+? h2o , what is the maximum amount of hco2h (46.0254 g/mo...
Questions

Mathematics, 28.04.2021 17:40

Mathematics, 28.04.2021 17:40

Biology, 28.04.2021 17:40


Mathematics, 28.04.2021 17:40





Medicine, 28.04.2021 17:40

Mathematics, 28.04.2021 17:40

Social Studies, 28.04.2021 17:40


Physics, 28.04.2021 17:40

Mathematics, 28.04.2021 17:40


Biology, 28.04.2021 17:40