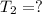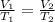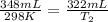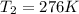Chemistry, 11.07.2019 18:30 maggiemae3645
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

You know the right answer?
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when...
Questions





Mathematics, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

History, 07.07.2019 13:00


Mathematics, 07.07.2019 13:00




English, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00


Mathematics, 07.07.2019 13:00

Social Studies, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

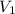 = 348 mL
= 348 mL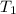 =
= 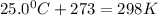
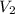 = 322 mL
= 322 mL