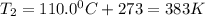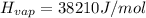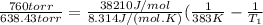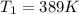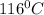Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1
You know the right answer?
Find the boiling point temperature at 760 torr of an isomer of octane, c8h18, if its enthalpy of vap...
Questions





Chemistry, 04.12.2021 01:00


Business, 04.12.2021 01:00












History, 04.12.2021 01:00

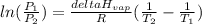
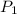 is the vapor pressure at boiling point = 760 torr
is the vapor pressure at boiling point = 760 torr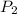 is the vapor pressure at T_{2} =638.43 torr
is the vapor pressure at T_{2} =638.43 torr