

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2
You know the right answer?
A0.99 m aqueous solution of an ionic compound with the formula mx has a freezing point of -2.6 ∘c ....
Questions

Mathematics, 09.06.2021 23:40

Mathematics, 09.06.2021 23:40


English, 09.06.2021 23:40

Mathematics, 09.06.2021 23:40

English, 09.06.2021 23:40

Mathematics, 09.06.2021 23:40

Engineering, 09.06.2021 23:40

Mathematics, 09.06.2021 23:40


Physics, 09.06.2021 23:40

Mathematics, 09.06.2021 23:40

Mathematics, 09.06.2021 23:40



Mathematics, 09.06.2021 23:40




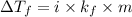 -(1)
-(1)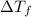 is depression in freezing point,
is depression in freezing point, is Van't Hoff factor,
is Van't Hoff factor,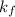 is molal freezing point depression constant, and
is molal freezing point depression constant, and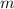 is molality of the solution.
is molality of the solution.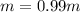 (given)
(given)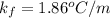
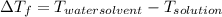
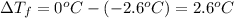
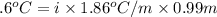
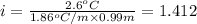
 is 1.412.
is 1.412.


