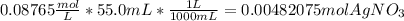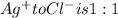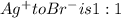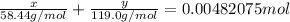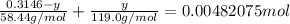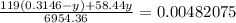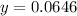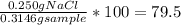Chemistry, 11.07.2019 19:00 alliwkatring
A0.3146-g sample of a mixture of nacl(s) and kbr(s) was dissolved in water. the resulting solution required 55.00 ml of 0.08765 m agno3(aq) to precipitate the cl–(aq) and br–(aq) as agcl(s) and agbr(s). calculate the mass percentage of nacl(s) in the mixture.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
A0.3146-g sample of a mixture of nacl(s) and kbr(s) was dissolved in water. the resulting solution r...
Questions




Mathematics, 08.04.2021 18:10




History, 08.04.2021 18:10


English, 08.04.2021 18:10


Mathematics, 08.04.2021 18:10

Mathematics, 08.04.2021 18:10




Mathematics, 08.04.2021 18:10

Mathematics, 08.04.2021 18:10

Mathematics, 08.04.2021 18:10



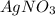 =
=