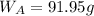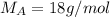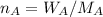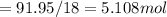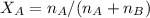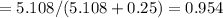A8.05 % ch3oh(aq) has a density of 0.976 g/ml at 18°c what is the mole fraction of the solvent in the solution? 0.953 8.05 91.95 0.0469
2) an aqueous solution of cesium chloride is prepared by dissolving 52.3 g cesium chloride in 60.0g of water at 25°c. the volume of this solution is 63.3 ml . what is the molality of the solution?
0.311 m
4.91 m
2.77 m
5.18 m
3) an aqueous solution of cesium chloride is prepared by dissolving 52.3 g cesium chloride in 60.0g of water at 25°c. the volume of this solution is 63.3 ml . what is the molarity of the solution?
4.91 m
2.69 m
5.18 m
2.77 m
4) the vapor pressure of ethanol, c2h5oh is 100.0 torr at 35 °c. calculate the vapor pressure of the solution formed by dissolving 28.8 g of alpha naphthol, c10h8o, in
36.8 g of c2h5oh. assume alpha naphthol to be nonvolatile at this temperature.
20.0 torr
43.9 torr
80.0 torr
56.1 torr
5) both ethanol, c2h5oh and propanol, c3h7oh, are volatile. at 35 °c, the vapor pressure of pure ethanol is 100 torr and that of propanol is 37.6 torr. what is the vapor pressure at this temperature of a solution is formed by mixing 36.9 g of ethanol and 12.0 g propanol.
15.3 torr
50.1 torr
84.7 torr
87.5 torr
the boiling point of pure ethanol, c2h5oh, is 78.4 latex: ^\circ ∘ c. its boiling point elevation constant is 1.22 °c/m. what is the boiling point of a solution formed by dissolving 8.00 g of alpha-naphthol (c10h7oh) in 100.0 g ethanol.
91.3 degrees centigrade
79.1 degrees centigrade
97.6 degrees centigrade
78.5 degrees centigrade
the freezing point of ccl4 is -22.92°c. calculate the freezing point of the solution prepared by dissolving 17.5g of pyrazine (c4h4n2) in 1250g of ccl4. the freezing point depression constant for ccl4 is 29.8 °c/m.
-22.50
-23.34
-17.71
-28.13
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of osmotic pressure
c < b < a < d
a < d < c < b
d < a < c < b
a < c < b < d
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of freezing point. the freezing point of pure water is 0.00 ∘ c and its freezing point depression constant is 1.86 ∘ c/m
d < a < c < b
b < c < a < d
c < b < a < d
a < c < b < d
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of normal boiling point. the normal boiling point of pure water is 100.00 ∘ c and its boiling point elevation constant is 0.512 ∘ c/m
c < b < a < d
d < a < c < b
a < c < b < d
b < c < a < d
a solution is prepared by dissolving 1.22 g of compound in enough water to make up 262 ml in volume. the osmotic pressure of the solution is found to be 30.3 torr at
35.0 °c. calculate the molar mass of the compound.
257 g/mol
2950 g/mol
3.88 g/mol
44.7 g/mol
i tried to solve them all but i keep getting wrong answers can anyone

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
A8.05 % ch3oh(aq) has a density of 0.976 g/ml at 18°c what is the mole fraction of the solvent in th...
Questions

Mathematics, 10.02.2021 03:50





Health, 10.02.2021 03:50

English, 10.02.2021 03:50


Mathematics, 10.02.2021 03:50


Social Studies, 10.02.2021 03:50

Spanish, 10.02.2021 03:50

Mathematics, 10.02.2021 03:50

Mathematics, 10.02.2021 03:50

English, 10.02.2021 03:50

World Languages, 10.02.2021 03:50


Mathematics, 10.02.2021 03:50