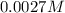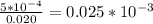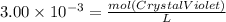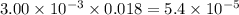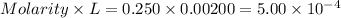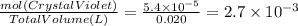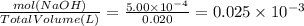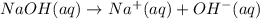Chemistry, 11.07.2019 21:30 aprilstalder
Asample was prepared by mixing 18. ml of 3.00 x 10^-3 m crystal violet (cv) with 2.00 ml of 0.250 m naoh. calculate the resulting concerntattions of cv and oh-

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Asample was prepared by mixing 18. ml of 3.00 x 10^-3 m crystal violet (cv) with 2.00 ml of 0.250 m...
Questions


Mathematics, 30.01.2020 20:55








Mathematics, 30.01.2020 20:55


Social Studies, 30.01.2020 20:55

Mathematics, 30.01.2020 20:55


Mathematics, 30.01.2020 20:56

Mathematics, 30.01.2020 20:56

History, 30.01.2020 20:56



Mathematics, 30.01.2020 20:56

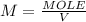
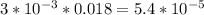
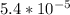
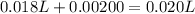
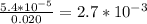 .
.