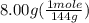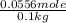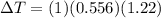Chemistry, 11.07.2019 23:00 kylucienne
The boiling point of pure ethanol, c2h5oh, is 78.4 ∘c. its boiling point elevation constant is 1.22 °c/m. what is the boiling point of a solution formed by dissolving 8.00 g of alpha-naphthol (c10h7oh) in 100.0 g ethanol. 91.3 °c 79.1°c 97.6 °c 78.5°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
The boiling point of pure ethanol, c2h5oh, is 78.4 ∘c. its boiling point elevation constant is 1.22...
Questions

Biology, 13.04.2021 17:00



Health, 13.04.2021 17:00

Mathematics, 13.04.2021 17:00


Mathematics, 13.04.2021 17:00






History, 13.04.2021 17:00




Mathematics, 13.04.2021 17:00



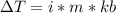
 is the elevation in boiling point
is the elevation in boiling point