Chemistry, 11.07.2019 23:30 eldercunningham
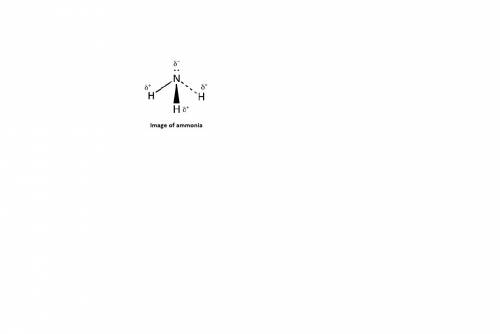
The electronegativity of nitrogen (n) is 3.0, while the electronegativity of hydrogen (h) is 2.1. knowing this, consider how the electrons will be shared in ammonia (nh3). what do you predict about the polarity of ammonia? a. each h atom has a partial positive charge

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
The electronegativity of nitrogen (n) is 3.0, while the electronegativity of hydrogen (h) is 2.1. kn...
Questions




Mathematics, 21.09.2020 14:01



Mathematics, 21.09.2020 14:01

English, 21.09.2020 14:01

Computers and Technology, 21.09.2020 14:01

Social Studies, 21.09.2020 14:01

Mathematics, 21.09.2020 14:01


Advanced Placement (AP), 21.09.2020 14:01



English, 21.09.2020 14:01