
Chemistry, 11.07.2019 23:30 steffweikeloyrt00
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of coffee with a temperature of 88.3 degrees c. assume the specific heats of coffee and milk are the same as for water (c = 4.19 j/g•c), and disregard the heat capacity of the container.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of co...
Questions



Mathematics, 20.07.2019 11:00

Mathematics, 20.07.2019 11:00

Mathematics, 20.07.2019 11:00

Biology, 20.07.2019 11:00


Mathematics, 20.07.2019 11:00


History, 20.07.2019 11:00

Mathematics, 20.07.2019 11:00



English, 20.07.2019 11:00

Mathematics, 20.07.2019 11:00


English, 20.07.2019 11:00



Mathematics, 20.07.2019 11:00

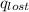 =
=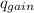 ,
, 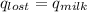 +
+ 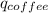
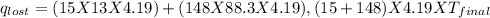 =(15X13X4.19)+(148X88.3X4.19)
=(15X13X4.19)+(148X88.3X4.19)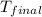 = 81.37 ° C
= 81.37 ° C

