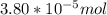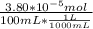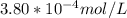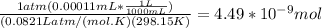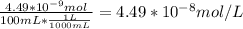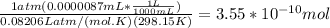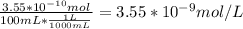A) a concentration of 5.24 ppm he means 5.24 μl of he per liter of air. using the ideal gas law in find how many moles of he are contained in 5.24 μl at 25.00°c (298.15 k) and 1.000 atm. this number is the molarity of he in the air. (2.11x10-7m) (b) find the molar concentrations of ar, kr, and xe in air at 25°c and 1 atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
A) a concentration of 5.24 ppm he means 5.24 μl of he per liter of air. using the ideal gas law in f...
Questions




Mathematics, 28.10.2019 15:31


History, 28.10.2019 15:31

Social Studies, 28.10.2019 15:31

English, 28.10.2019 15:31

History, 28.10.2019 15:31

Biology, 28.10.2019 15:31

History, 28.10.2019 15:31




Biology, 28.10.2019 15:31

Mathematics, 28.10.2019 15:31

Chemistry, 28.10.2019 15:31



Mathematics, 28.10.2019 15:31

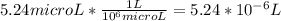
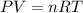
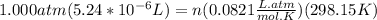
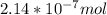
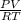
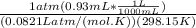 =
=