
Chemistry, 12.07.2019 02:00 keviongardner
An aqueous solution that is 50.0 percent sulfuric acid (h2so4) by mass has a density of 1.143 g/ml. determine the molality of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
An aqueous solution that is 50.0 percent sulfuric acid (h2so4) by mass has a density of 1.143 g/ml....
Questions


Chemistry, 16.05.2021 06:40

Mathematics, 16.05.2021 06:40

Mathematics, 16.05.2021 06:40

Mathematics, 16.05.2021 06:40

History, 16.05.2021 06:40


Mathematics, 16.05.2021 06:40

Chemistry, 16.05.2021 06:40

Mathematics, 16.05.2021 06:40





Mathematics, 16.05.2021 06:50

Mathematics, 16.05.2021 06:50




Mathematics, 16.05.2021 06:50

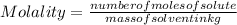 - (1)
- (1)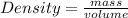 - (2)
- (2)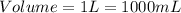
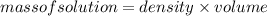
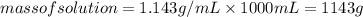
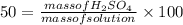
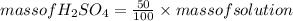
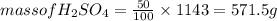
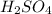 :
: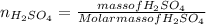
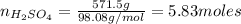
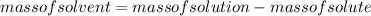
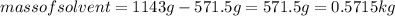
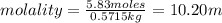
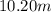 .
.

